SPEVIGO® (spesolimab) safety profile

safety profile
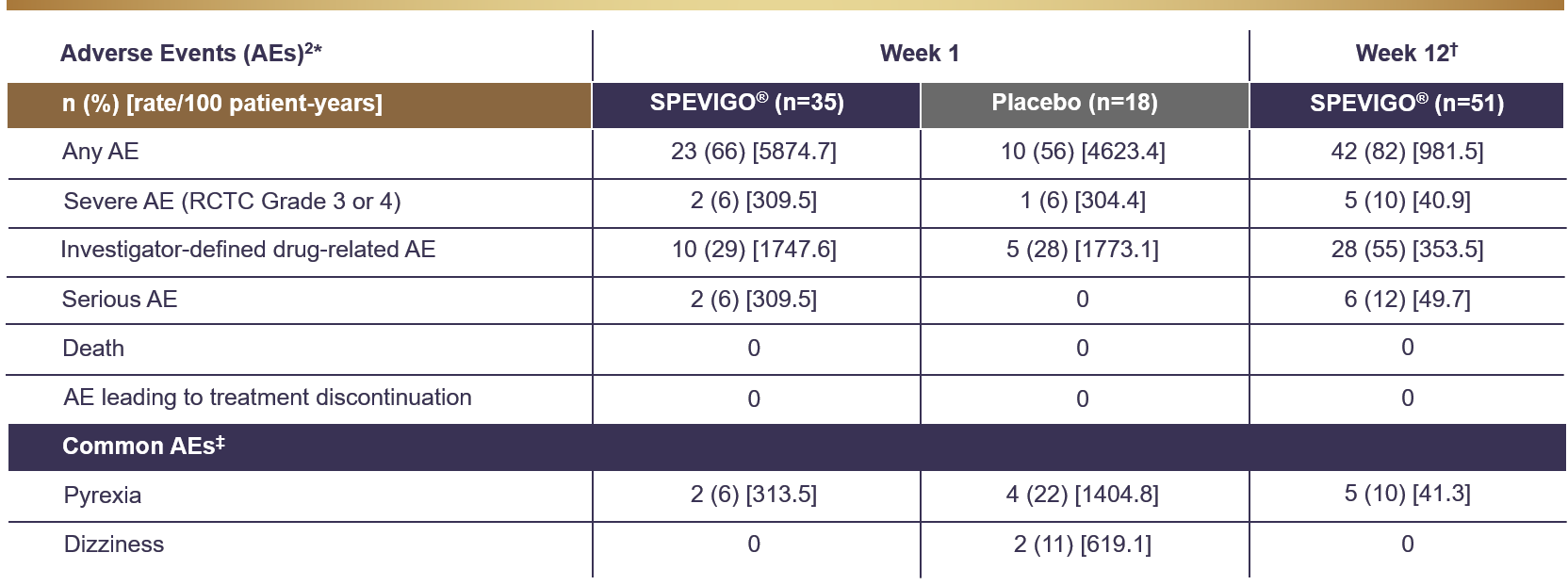
SPEVIGO® Summary of adverse events1,2
|
All AEs occurring between the start of treatment and end of the residual effect period (16 weeks after the placebo dose or last dose of SPEVIGO®. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) v23.1. AE severity was graded according to the RCTC v2.0. Pustular psoriasis was excluded as an AE from this safety analysis.2
†Data set at Week 12 included patients randomised to SPEVIGO® who received up to 3 doses of SPEVIGO® and patients randomised to the placebo group who received OL SPEVIGO® at or after Day 8. All AEs in the residual effect period are included but censored at the day rescue treatment with SPEVIGO® was administered.2
‡Common AEs are reported in ≥10% of patients in any treatment group.2
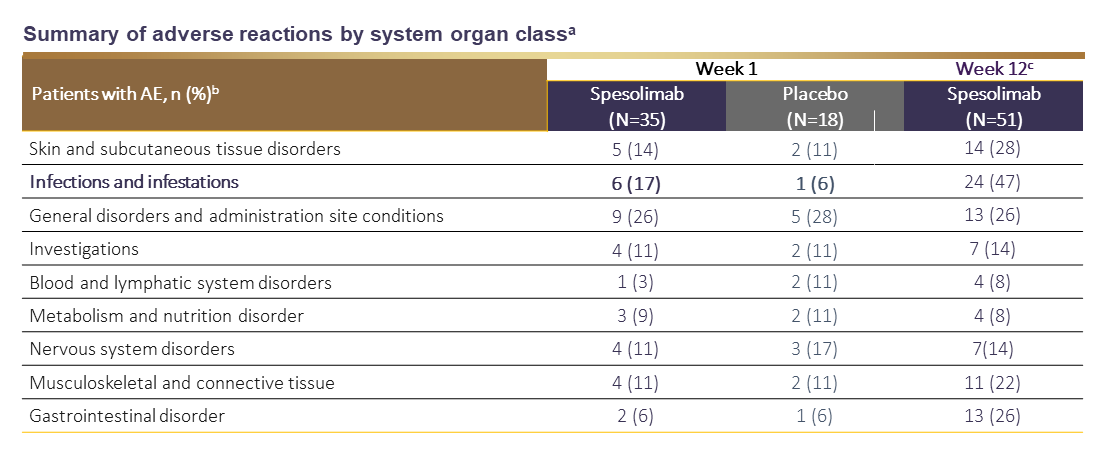
SPEVIGO® Summary of adverse reactions1,2
|
SPEVIGO® may increase risk of infections. During the 1-week placebo-controlled period in the EffisayilTM 1 trial, infections were reported in 17.1% of patients treated with SPEVIGO® compared with 5.6% of patients treated with placebo.1
a AEs by system organ class are reported in ≥ 10% in any treatment group.
b AEs that occurred between the start of spesolimab or placebo administration and the end of the residual-effect period (16 weeks after last dose of treatment).
c Dataset at Week 12 includes patients randomized to spesolimab and patients initially randomized to placebo who received open-label spesolimab at Day 8.
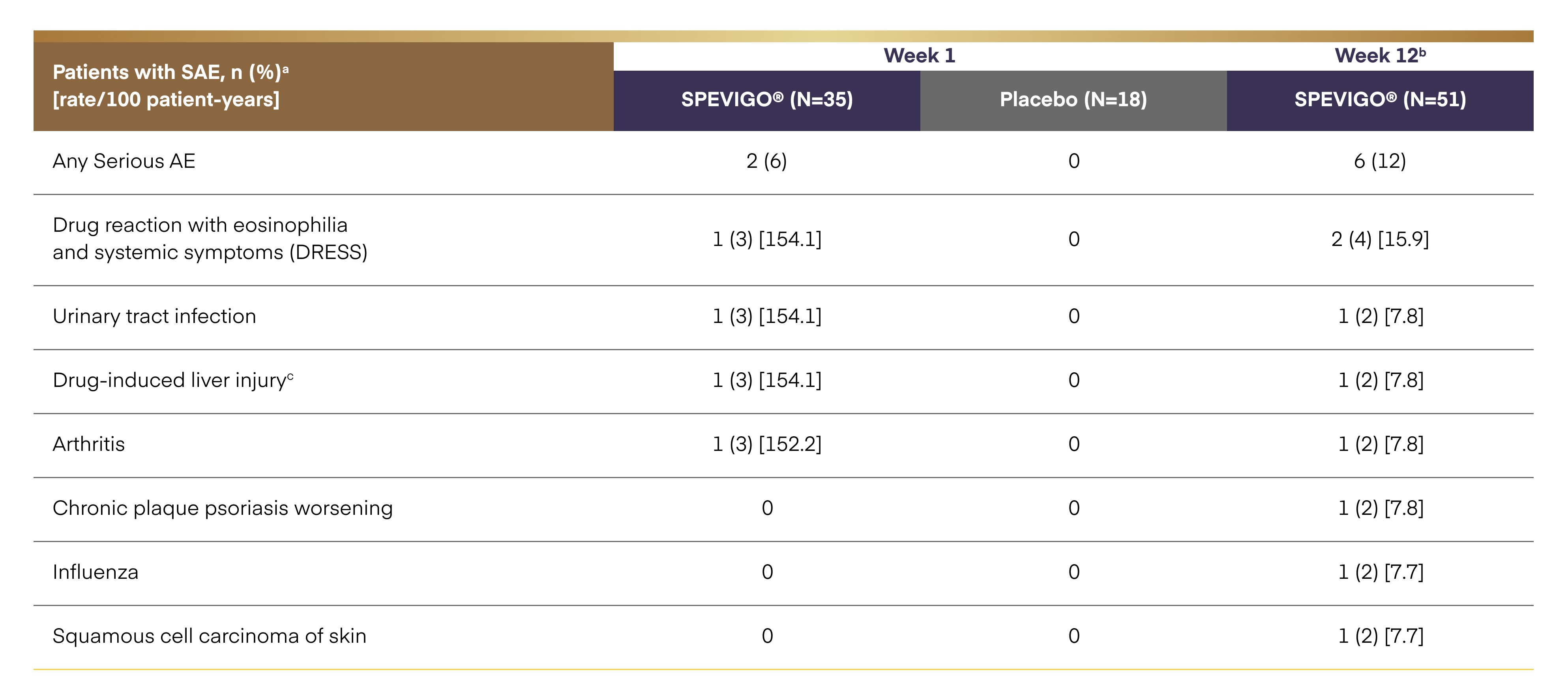
Serious adverse events2
|
References
-
SPEVIGO® Summary of Product Characteristics. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
-
Supplementary Appendix: Bachelez H et al. N Engl J Med. 2021;385(26):2431-2440. doi:10.1056/NEJMoa2111563
-
Bachelez H et al. N Engl J Med. 2021;385(26):2431-2440. doi:10.1056/NEJMoa2111563
Spevigo® (spesolimab), interleukinhämmare, 450 mg koncentrat till infusionsvätska, lösning. Rx. EF.
▼Detta läkemedel är föremål för utökad övervakning.
Indikationer: behandling av skov hos vuxna och ungdomar från 12 års ålder med generaliserad pustulös psoriasis (GPP) som monoterapi. Kontraindikationer: Kliniskt relevanta aktiva infektioner (t.ex. aktiv tbc). Varningar och försiktighet: Kan öka infektionsrisk. Vid överkänslighetsreaktioner, pausa infusionen för att hantera reaktionen. Uppmärksamma symtom på nydebuterad perifer neuropati. Samtidig användning med andra immunsuppressiva medel rekommenderas inte. Beakta intervall mellan vaccination med levande vaccin och behandling. Bör undvikas under graviditet. Boehringer Ingelheim AB, tel 08-721 21 00. För ytterligare information samt priser se www.fass.se. Senaste översyn av produktresumén: 09/2024.
PC-SE-103623_March 2025
